The other day I very excitedly set about my first woad vat of the summer. There were lots of supplies and equipment to assemble, dirty 5 gallon buckets to wash, yarns to soak. I got everything ready, and harvested seven and a half pounds of leaves from my glorious woad beds. You can tell from the title of my post that I made a big mistake along the way, but I hope to turn it into a learning opportunity for others, so I relate the tale….
Here are the bags of leaves. They were very clean because we have had practically no rain. Sometimes the leaves are muddy so I rinse them off, but not this time.
 Next I shred the leaves up by hand. Normally I grab a fistful and rip the leaves into several pieces. You can see the blue staining in the stems below. This doesn’t always happen. Sometimes I don’t notice any “breaking blue” or “bruising blue” but still get good color from a vat.
Next I shred the leaves up by hand. Normally I grab a fistful and rip the leaves into several pieces. You can see the blue staining in the stems below. This doesn’t always happen. Sometimes I don’t notice any “breaking blue” or “bruising blue” but still get good color from a vat.
The five gallon bucket gradually fills with shredded leaves.
Meanwhile in the kitchen I am boiling two large pots of water. If I do not have such a big harvest of leaves, I use a smaller bucket for reasons I will explain shortly.
I pour the pots of boiling water over the leaves. Careful of your feet if you’re barefoot! Now the leaves are ready to steep, or extract. At this point in the process you need to exclude as much air as possible. So I fill the bucket to the top with water, and then press down the lid until water squirts out. Use oven mitts, insulated gloves, or a towel to protect your hands from the hot water.
This way I am pretty sure that there’s no air left at the surface of the bucket. Then the leaves steep for about 45 minutes or an hour.
When I take the lid off the leaves are wilted and have turned very dark.
The extract is a rich color sort of like port. I strain out the leaves, squeeze out as much of the juice as I can, and pour it back into the vat.
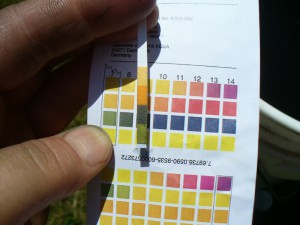
Next I add ammonia to make the vat alkaline. Depending on how fresh the ammonia is, the amount can vary, so I test with pH strips. For woolen yarns, which are damaged by high pH, I aim for 9 or 10. You can go higher with cellulose. The color of the vat changes dramatically from clear reddish-brown to murky dark green as I add the ammonia.
Check the pH. The staining on my fingers is from the leaves. Again, this doesn’t always happen.
At this point I got distracted. The next step ought to be aerating the vat, which involves pouring the alkaline vat liquid back and forth between two or three buckets for about 5 minutes to incorporate oxygen. But I wanted Matthew to take a picture of me doing it because I couldn’t hold the camera and pour a bucket of hot woad vat liquid at the same time. And I wanted to boil water to dissolve the thiox I planned to use.
Thiox, or thiourea dioxide, is a reducing agent. In terms of its role in the woad vat, this means it uses up all the unbonded oxygen in the vat liquid. If I only have a small quantity of leaves, I use a smaller bucket because all the extra water that it takes to fill a five-gallon bucket isn’t really helping. It just means you have a greater volume of liquid which must be made alkaline and then reduced, which seems like a waste of supplies to me.
For many years I used RIT Color Remover, which contains sodium hydrosulfite, as my reducing agent, but nowadays the smell of the perfume bothers me. Which is sort of ironic because this process is very smelly. Woad itself is a strong-smelling plant, not to mention boiled woad, and ammonia…. Anyway. I was thinking about boiling water, and needing a jar to dissolve it in.
So I went inside, found a jar, got the thiox measured and dissolved, then came back outside and immediately added it to the vat. I did not realize until later that I had skipped the crucial step of aerating.
After you add your reducing agent, you put the lid back on and wait for the vat to reduce. This takes about 45 minutes or so. When the alarm went off, I opened the lid and was very surprised by the color. On the surface it looked OK, and there was some blue around the edges.
But the liquid itself was clear, bright yellow.
When things are all going to plan, the reduced vat is anywhere from a greenish yellow to an olive green. Clear yellow usually means the vat is over-reduced, so I stirred it a little to add some oxygen back in. No change. I was confused but still didn’t realize what had happened. I added a pre-soaked skein of rug wool to the vat and worked it under the surface a little, then left it for 10 minutes.
When I took it out, my yarn did the usual color-shift from yellow to blue.
I was expecting a lot of color from this vat because of the staining on my fingers. But it was a very very pale blue. Something was wrong. This is when I realized with a horrible mental clanging noise that I hadn’t aerated the vat. I dyed another skein of rug wool very pale blue, and as skein of 2/8 wool a weirdly uneven grayish blue. Then I gave up in disgust.
Here are the three pale skeins, and on the far left is a more typical single-dip woad blue from a vat I did last fall.
However, this mishap is a good opportunity to explain the chemistry of a woad vat, and why aeration in particular is so important. When you extract the leaves, you are extracting the indoxyl which is in the leaves. Indoxyl is the precursor to indigotin. Indigotin is the molecule that makes fibers blue. Indoxyl is only soluble in an alkaline solution, hence the ammonia. You can also use other things to make your vat alkaline, such as a wood ash solution or lye. To convert the indoxyl to indigotin, you need some oxygen. Obviously there is some oxygen in the air and some in the vat liquid, but if you do not aerate the vat, you do not supply enough oxygen for the indigotin molecules to form. This was my problem.
If you extract your woad leaves, create an alkaline solution, and aerate, you should have a lot of indigotin molecules in your vat just waiting to turn things blue. But not quite yet. The blue form of indigotin is not water soluble. In fact, if you leave it alone it will settle down to the bottom of the vat. To dye fiber you need to reduce the vat. You can do this in a lot of different ways. Traditionally, fermentation reduced the vat; bacteria consumed the available oxygen over a period of days or weeks. I have had some funny but not very successful experiences with urine and yeast vats, but a faster way is to use a reducing agent. I am a fan of the chemically-assisted method outlined by Rita Buchanan in A Weaver’s Garden and A Dyer’s Garden (published by Interweave Press, but they no longer have it on their website). The reducing agent ties up oxygen, hydrogen is liberated, and the indigotin converts to a “white” form called leuco-indigotin. In this state it is dissolved and where ever the vat liquid can penetrate your fiber, the leuco-indigotin will also travel. If it is not reduced, the indigotin will still be there, but it will have a much harder time getting onto your fiber and you will get blotchy results.
When your vat is reduced, you dip your fiber into the vat. Try not to add any more oxygen at this point or the leuco-indigotin will convert back to the blue state. For the first dip you can leave it as long as you like. I usually do between 10 and 30 minutes (unless I am overdyeing something to make green then it’s usually just a few minutes). Then you pull out your fiber and a magical transformation occurs. The fiber will look yellow, then as the leuco-indigotin is exposed to the oxygen molecules in the air, it will convert to the blue form of indigotin and gradually your yarn will turn blue. Then you let it oxidize for at least as long as you dipped it, the longer the better. Opening up the strands of wool so the air can get in helps with even color.
If you want to get darker shades of blue, you can dip your yarn or fiber in again. However, this gets tricky. The fact that the vat is reduced means that some of your blue indigotin will be dissolved when you put it back into the vat. So, subsequent dips should be very short. I have found that it actually works best to dip a skein once in a vat, and then let it air, dry, and get rinsed. Then I wait until my next vat to dip it again. I almost always do a delayed rinse for all my dyeing, which means I let the yarn dry completely before I rinse it. I think this helps with the fastness of the color. If your wool has been exposed to an alkaline solution like a woad vat, rinse it in a vinegar solution to neutralize it.
Hopefully I will post about a successful woad vat later this week.

















One thought on “Woad Vat Mishap”
Comments are closed.